Ammonium Chloride and Sodium Hydroxide Net Ionic Equation
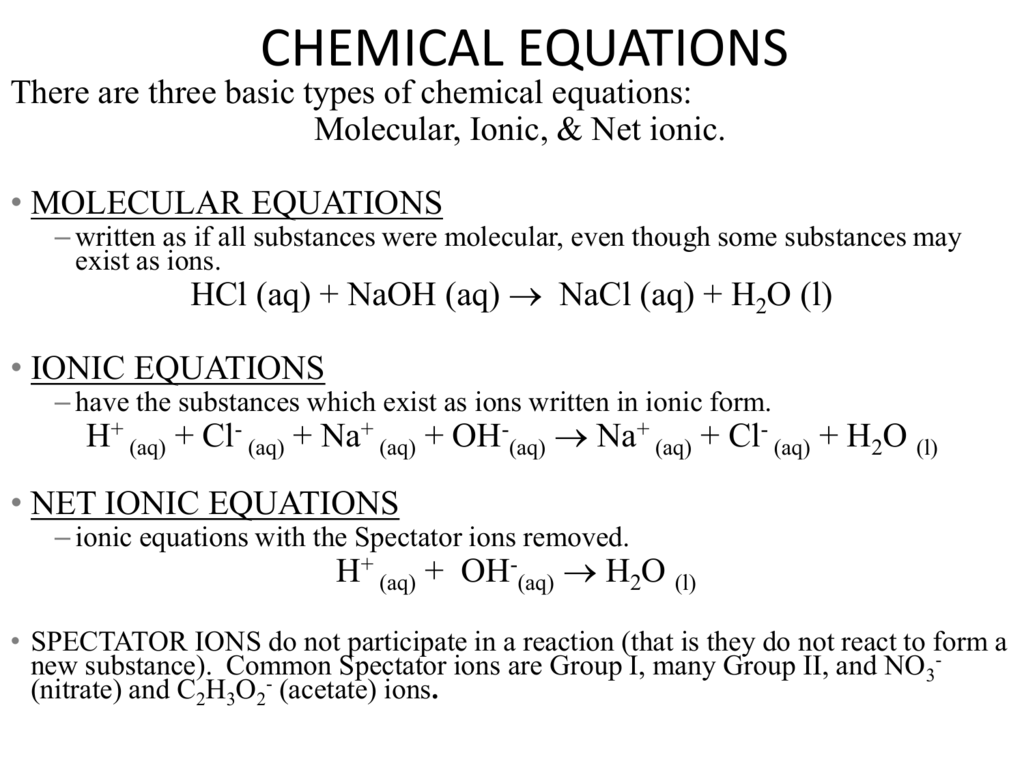
For example when sodium hydroxide reacts with hydrochloric acid sodium chloride is formed. NaOHaq HClaq NaClaq H 2 Ol In general such neutralization reactions are represented by one simple net ionic equation.

How To Write The Net Ionic Equation For Nh4cl Naoh Nacl H2o Nh3 Youtube
Sodium hydroxide reacts with protic acids to produce water and the corresponding salts.

. Complete and balance the equation. Write the equation for the reaction that occurs when a drop of water is added to products. Potassium chloride sodium carbonate see results Add 1-2 ml of a 01 M potassium chloride solution to a test tube containing 1-2 ml of a 01 M sodium carbonate solution.
OH aq H aq H 2 Ol.

How To Write The Net Ionic Equation For Nh4cl Naoh Nacl H2o Nh3 Youtube

How To Write The Net Ionic Equation For Nh4no3 Naoh Nano3 Nh3 H2o Youtube
No comments for "Ammonium Chloride and Sodium Hydroxide Net Ionic Equation"
Post a Comment